This article is written by Surupa Hossain Bhuiyan, pursuing a Diploma in Cyber Law, Fintech Regulations and Technology Contracts from Lawsikho.com.
Table of Contents
Introduction
Three-dimensional (3D) printing is the most widely used colloquial term for the scientific term’ additive manufacturing.’ Inventor Wyn Kelly Swains first patented the additive manufacturing process in 1977, but Chuck Hull, who invented the stereolithographic file format to digitize the process, began selling this process (introduced the first commercial 3D printer) in1984. In the 1990s, the 3D printer was first used for dental implants and custom prosthetics in the medical field, since then 3D printing has advanced and has been economically accessible.
According to 3D Hubs’ 2020 3D Printing Trends Report, analysts expect the 3D printing market to grow at an average of 24 percent over the next five years, reaching $35 billion by 2024. The graph below (in Fig. 1) summarizes data reported by ten reputable market analysts who evaluated the additive manufacturing market segment in 2019.
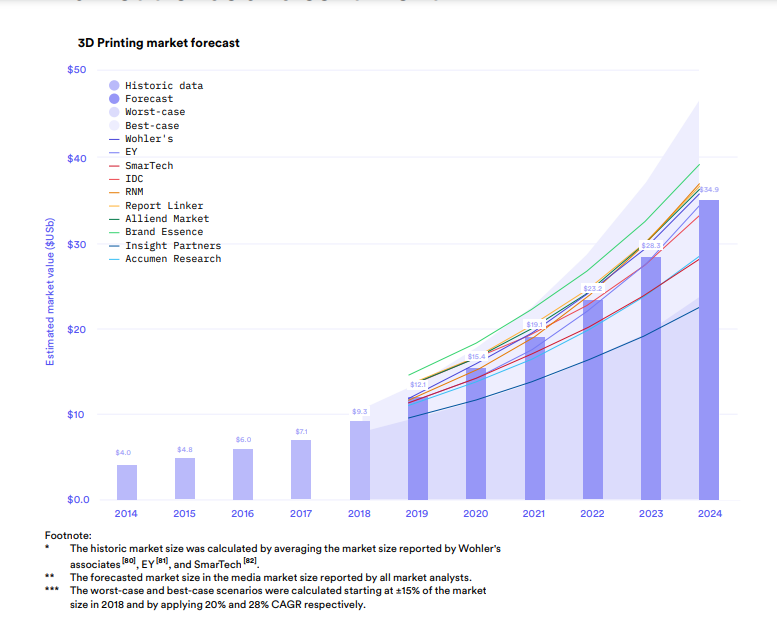
Fig1. 3D Printing Market Forecast from 3D Hubs
What is 3D printing?
3D printing is a process where the material is joined, usually layer upon layer (three-dimensional structure through a serial compound, layering of “ingredients” such as powders, metal, polymers, bio-material, ink, etc.), to make an object from 3D-model data, as opposed to subtractive manufacturing. The objects from 3D printing are produced from a digital file, rendered from a computer-aided design (CAD) drawing (the blueprint) or a Magnetic Resonance Image (MRI).
The CAD file is a computer file that contains a 3D digital representation of the design to be produced. CAD files are created using CAD software programs, which allow users to design and manipulate shapes into 3D models of objects. These design files act as blueprints that direct the printer to build the tangible output (the design is held in a CAD file). Design content can easily be distributed, shared, and transferred on a global scale, like any other computer file, due to the digital nature of the CAD files. Since this manufacturing method does not rely on molds, it is possible to rapidly modify various pieces of specialized equipment and designs.
How is 3D printing used and for what
A typical 3D printing workflow includes the following steps: (1) Image acquisition; (2) File manipulation; (3) 3D Print; (4) Post-processing and polishing and (5) Validation and quality control.
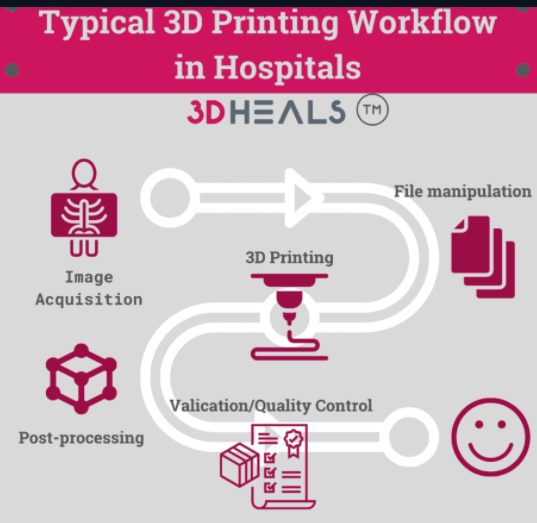
Fig2. A typical workflow for 3D printing in Hospitals from 3D Hubs
3D printing technology is creating incredible potential in the medical industry and its implementation is rising. To build medical devices such as prosthetics, dental implants, surgical implants, hearing aids, and bone grafts, 3D printing technology has already been used. Consequently, in bio-printing (a special form of 3D printing that uses cells or tissue as a medium in the printing process, instead of plastic or metal) and regenerative medicine, ophthalmology and pharmaceutics, 3D printing technology has been used too.
Benefits of 3D Printing
Among several advantages, additive manufacturing is mainly beneficial in easily providing point-of-care services to patients, as customized digital blueprinting can be sent to the on-site 3D printer. For example, recently, an Italian 3D printing start-up Isinnova has saved the lives of Covid-19 patients after developing a replacement ventilator valve when supplies at Chiari hospital in Brescia ran dry and their regular supplier wasn’t able to provide the devices in time due to the pandemic. The fascinating fact was that Cristian Fracassi, the founder & CEO of start-up Isinnova, along with his colleague (visited the hospital directly and inspected the valves themselves and rapidly created a prototype), delivered a 3D printer directly to the hospital and was able to redesign and produce a new 3D valve piece within just six hours and managed to produce 100 3D respirator valves in 24 hours (in an emergency without certification); (however, the founder is likely to face a potential legal action for infringement of intellectual property rights by the company that owns the oxygen valve patent).
In addition, new tools and therapeutic techniques developed through 3D printing bring new levels of comfort and personalization to the treatment of patients. And this newly available technology, too, allows doctors to gain a better understanding of complex cases and offers new tools that can ultimately lead to a higher standard of care.
Generally, traditional manufacturing methods are cheaper for large-scale production, but the cost of 3D printing is becoming competitive for smaller production runs, which is likely to save taxpayers millions of dollars. 3D printing will reduce production costs and manufacturing costs by increasing efficiency, maximizing resources, and eliminating outdated development procedures.
Disadvantages of 3D Printing
Despite its unique advantages, 3D printing does have potential drawbacks, primarily as a result of the relatively early stages of this technology. Even the top printers have slower build rates and higher production costs than those for mass production parts. Also, additive manufacturing introduces different variables into the manufacturing process that could affect the mechanical and geometric properties of the finished part. For example, in powder-bed printing (a type of additive manufacturing that uses powder—often for metals—as the material that is then heated and fused together layer by layer), concerns arise that powder residue may remain from one print to the next or that separate material powders could cross-contaminate and result in structural defects. In addition, the volatile nature of some printing raw materials adds new hazards to the manufacturing process, particularly when located outside of traditional manufacturing facilities.
Legal issues that emerge from the application of 3D printing within the medicine and healthcare field
3D printing revolutionizes the manufacture of products, the materials used, and who is the manufacturer. Not only does this technology stimulate the medical and healthcare industry, but also the legal landscape (Fig 3). While it is not clear how the law will change, in the absence of a precedent, it still needs to be adapted or modified when 3D printing becomes normal at this stage. A wide range of legal fields will be affected by 3D printing, including product liability, regulatory, intellectual property, privacy/digital security, environment, transportation, communications, imports, and exports, etc. This blog will therefore focus on a few legal issues.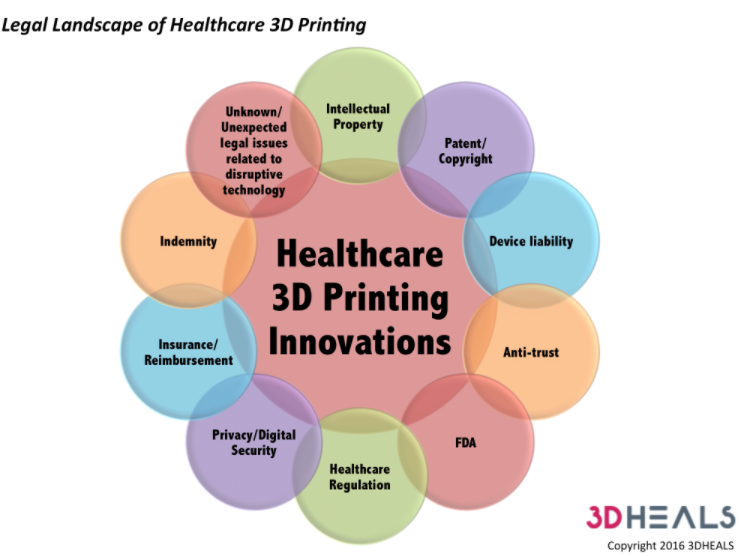
Fig3. Legal Landscape of Healthcare 3D Printing by 3D HEALS infographic
I. Regulatory Issues
3D printing allows doctors to print drugs with doses customized for each patient and surgeons to practice complex surgery beforehand by 3D printing prototypes of the patients’ organs. The existing regulatory framework to some extent adequate for the regulation of drugs and medical devices but there are areas of the law which might require changes to accommodate 3D printed drugs and medical devices.
As of now, no 3D printing law has been introduced to date, there is a concern about how 3D printing and 3D printed products can be regulated. All legal entities, including hospitals and end manufacturers, are likely to face new and unknown challenges with 3D printing compliance and liability protection as there is no guidance on care printing or bio-printing problems.
There are also a number of procedures and safeguards to be considered in the event of point of care printing. In order to ensure compliance with CAD files, printers, software, and material warnings and instructions, point of care manufacturers should consider adopting procedures and quality control. A “post-manufacture” inspection process and controls designed to verify that a product was printed as intended and in compliance with the instructions will also need to be considered.
Similarly, a question that arises is whether 3D printed drugs need to be specifically approved (by a governing body) because of a change in the production and manufacturing process. While, it is disputed whether downloading CAD files of drugs will amount to “import”, or whether the printing activity would be separately construed as ‘manufacture’ as without any physical movement of goods when the 3D printing enables full schematics of drugs to be imported electronically.
In the case of medical devices, if the modification affects the quality of the device, a change in the manufacturing process, equipment or testing is considered to be a major design change. Before making the change, the manufacturer or importer of the medical device is required to obtain permission from the competent authorities. Therefore, manufacturers and importers of the approved medical device may be required to obtain prior approval from the medical device regulator if they wish to print a medical device in 3D that was previously manufactured by other means.
II. Product liability Issues
Although product liability laws in the traditional design and distribution chains are well established, the uniqueness of 3D printing leads to many challenges and unknowns which do not fit the current legal landscape.
Product liability is defined as the liability which may be levied on any or all of the parties in the manufacturing and supply chain of such goods for damage or injury caused by a product. Three theories of product liability are primarily available-warranty, negligence, and strict liability. Eventually, these three theories would be relevant in the medical 3D printing area to protect the patient from injuries that are inferable from faulty products.
The question is how 3D printed medical devices or products can be handled as they have infinite possibilities under product liability law. Any physical object produced by 3D printing seems to fit under conventional product definitions, but when considering the three main theories of product liability law, the possible problems and effects on product liability are uncertain and limitless.
-
Product
What emerges from the 3D printer is operated by a software code to work the printer; 3D printing requires CAD files, whether this software would also be considered a product is questionable. Purely electronic data, such as code, does not constitute a product. Computer software is commonly characterized as a service rather than a product (but it is a product when the maker of the software sells the software as a product when licensing it to a business needs).
-
Defectiveness of Product
In case the 3D printed product is defective it is indistinct who will be liable for it – is it the maker of the CAD file, the maker of the 3D printer, the manufacturer of the material used or the consumers themselves (allocation of liability will become complex when the consumer prints the good). The liability can be allocated between either or all of the above parties depending on the facts and circumstances of the case. The prospect of point-of-care printing and mass production of 3D printing on the other hand raises unanswered legal questions involving potential design-defect claims.
- Strict Liability
It is not adequately addressed who will be liable in case injuries are caused due to the use of defective CAD software in the medical field. Since, strict liability will not be imposed upon hospitals as they act as service providers, not sellers of products, as they are neither affiliated with drug or device manufacturers nor marketers in the commercial sphere. For drugs or medical devices, the manufacturer need only warn a prescribing doctor about the risks, rather than an end-user. It is believed that hospitals and other healthcare providers are to provide services rather than to sell products.
Although the majority rule traditionally holds that hospitals are service providers not strictly liable for personal injuries arising from product defects that could change as hospitals start to incorporate a 3D printing center on-site. There is a case that suggests software can be a product for purposes of product liability. In Corley v. Stryker Corp[2014],6:13-CV-02571, the plaintiff, Ouita Corley, underwent a knee replacement surgery that involved the use of a “disposable, single-use cutting guide” that was intended to assist the surgeon during the course of the knee replacement procedure. The cutting guide was created from MRI or CT scans using 3D-imaging software to develop a surgical plan prior to surgery. Following the procedure, she experienced a range of issues and filed suit alleging that these problems were due to her surgeon’s use of the defective cutting guide. The court allowed the plaintiff’s products defect claim to go forward, finding that she had “sufficiently alleged that the cutting guide used during Ms. Corley’s surgery was unreasonably dangerous in design due to the alleged software defects.”
Ms. Corley has implications for the software used to create similarly customized 3D-printed medical devices, since both use patient-matched images. The courts may now be willing to stretch the definition of the product to include electronic files used to make bespoke 3D-printed objects, reasoning similarly to Corley that the file is part and parcel of the completed product and therefore subject to product liability laws. Thus, a court encountering 3D-printed products liability type claims may find that if the software or electronic file is defective, the entire system is defective.
- Legal challenges by a supplier and software providers
Likewise, suppliers of raw materials may also face legal challenges with their products being used for applications or in ways outside of what may have been tested or intended (powders, fibers, or other print materials). In addition, when negotiating supply contracts with manufacturers or users engaged in printing, material suppliers will want to consider indemnity and defense provisions, offering protection from the purchaser if the supplier is named in any lawsuits resulting from the use of the materials.
3D printers and software are two variables in the production process of 3D printed devices; hence it may be advisable to software providers (programmer/manufacturer) and 3D printer manufacturer to carefully consider warnings, instructions and contractual protections when entering the 3D printing market besides negotiating indemnity and defense provisions.
III. Intellectual Property Issues
For the intellectual property (IP) regime in general, 3D printers in the healthcare industry present important challenges as the different stages in the process from producing the CAD file to printing the final tangible product, will cause the application of various types of intellectual property. It is, therefore, necessary to distinguish between the protection of the CAD file and the protection of the printable object in order to address issues of intellectual property.
It is evident that 3D printing simplifies the manufacturing of medical devices but on the other hand, the concerns for counterfeiter products are emerging too. The current laws include protections for copyrighted materials, patented inventions, trademarks, and trade secrets for the manufacturing of medical devices in order to avoid the production of counterfeit and piracy products from 3D printing (though, the current IP laws were not designed to deal with the type of technology that exists today).
While the application of intellectual property law may be apparent in relation to 3D printer outputs, the role of the law is not as straightforward in relation to design files. CAD files, for example, maybe work in need of copyright law protection but the actual application of this is still unclear. However, legal scholars claim that CAD files should be treated as “artistic works” and should qualify for copyright law protection because they can be a technical drawing (including a diagram, map, chart, or plan.
The CAD file consists of data that is generated using CAD software. Interestingly, the software is protected by copyright law because of its “literary work” but data cannot be protected by copyright law (however, can be protected by trade secrets. For example, Magic Leap, a Florida-based startup 3D printing company, sued two of its former employees for trade secrets in 2016 Magic Leap Inc. v Bradski et al (2017) Case 5:16-cvb-02852.
-
Confidentiality & Privacy Issues
The healthcare provider needs to consolidate the personal data of an individual into the 3D printer with the consent to produce a 3D printed medical device according to the person’s need. Then again, for purposes such as research, marketing, and so forth, this information or 3D printed items may also be used (if authorized to). Therefore, in the medical sector, protecting the confidentiality and privacy of personal health data is crucial as breaches may trigger security and privacy laws.
The New York University, Tandon School of Engineering researchers discovered (in July 2016) that 3D printing raises cybersecurity threats in the manufacturing phase that could impact the final product’s reliability. The research found that without detection, a hacker could theoretically alter the orientation of the printer head, affecting the device’s intensity by as much as 25 percent. Alternatively, while it is linked to the internet, a hacker might exploit a 3D printer to insert internal defects while the product is being printed.
An article in the Washington Post recently highlighted the fact that medical devices themselves were somewhat not secure. There is a growing exposure of medical devices to hacking. Hence, the researchers in cybersecurity have also raised concerns about implantable medical device vulnerabilities that hackers might exploit to harm or even kill patients. Due to hacking concerns, former vice president Dick Cheney famously had his internal pacemaker taken offline.
Conclusion
3D printing with endless potential is like any other technology, exciting innovation, and is not without risks many of which are yet to be discovered. Without carefully considering the risks, it’s tempting to want to get ahead of the competition and jump into the healthcare market. There are many uncertainties about how in future 3D printing could lead to possible exposures to liability. Because Since 3D printing at the same time involves one or more separate areas of the law, it creates grey areas, making it more difficult to understand how the law will be applied or enforced. 3D printing opens up new doors to legal issues for law enforcement agencies to deal with.
LawSikho has created a telegram group for exchanging legal knowledge, referrals and various opportunities. You can click on this link and join:
https://t.me/joinchat/J_0YrBa4IBSHdpuTfQO_sA
Follow us on Instagram and subscribe to our YouTube channel for more amazing legal content.
 Serato DJ Crack 2025Serato DJ PRO Crack
Serato DJ Crack 2025Serato DJ PRO Crack









 Allow notifications
Allow notifications


